Nuclear Chemistry
I. What is nuclear chemistry?
a. Nuclear changes vs. chemical changes
i. A nuclear change is a change in which the nucleons (things in the nucleus) change. For instance, if the number of neutrons or protons in the nucleus changes, that is a nuclear change. A nuclear change often turns one element into another element. A nuclear change is a change that occurs within the atom.
1. Example of a nuclear change:
1n + 14N → 14C + 1H
(By the way: This equation shows the formation of the isotope carbon-14. Nitrogen-14 captures a neutron to produce C-14, which is the isotope used in carbon dating. More on this later.)
ii. A chemical change is a change in which atoms join together, split apart, or rearrange. A chemical change involves breaking or forming bonds between atoms.
1. Example of a chemical change:
CH4 + 2O2 → CO2 + 2H2O
(This just shows the burning of methane, which is basically the natural gas that we use in our Bunsen burners.)
II. Why do we care about nuclear chemistry?
a. Nuclear power – we can harness the energy stored in the powerful bonds between protons and neutrons to turn the lights on in our houses.
b. Nuclear weapons - we can harness the energy stored in the powerful bonds between protons and neutrons to destroy a city in less than one second.
c. Archaeology – we can use the fact that all living things contain radioactive carbon to determine the age of fossils. This is called carbon dating.
d. Radiochemistry and nuclear medicine – certain chemical reactions and lab tests make use of the fact that two different isotopes of the same element have the same chemical properties even though they have different nuclear properties.
e. Cosmochemistry - the study of the chemical composition of and changes in the universe
III. A review of atomic structure
a. Nucleus vs. electron cloud
i. In this chemistry course, we are concerned with only three subatomic particles: the electron, the proton, and the neutron.
ii. The protons and neutrons are located in the nucleus. The electrons are located outside the nucleus in the electron probability cloud.
iii. Protons and neutrons are not involved in ordinary chemical reactions.
b. Atomic number, mass number, and isotopes
i. The atomic number of an element is the number of protons.
1. The atomic number (Z) of an element is shown in the lower left-hand corner of the element’s symbol. The mass number (A) appears in the upper left-hand corner.
![]()
Examples:
a.
![]() The mass
number is 4, the atomic number is 2.
The mass
number is 4, the atomic number is 2.
b.
![]() The mass
number is 238, the atomic number is 92.
The mass
number is 238, the atomic number is 92.
c.
![]() The mass
number is 14, the atomic number is 6
The mass
number is 14, the atomic number is 6
d.
![]() The mass
number is 12, the atomic number is 6.
The mass
number is 12, the atomic number is 6.
2. The atomic number is redundant when the element symbol is given, so it is often omitted.
Examples of representing helium-4:
![]() He-4
He-4 ![]()
Examples of representing uranium-235:
![]() U-235
U-235 ![]()
ii. ALL atoms of a given element have the same number of protons.
iii. Atoms that have the same number of protons and neutrons are identical nuclides.
iv. Atoms that have the same number of protons but different numbers of neutrons are isotopes of the same element.
v. Atoms that have different numbers of protons are atoms of different elements.
c. Nuclear properties vs. chemical properties
IV. Nuclear Changes
a. Radioactive decay
i. Alpha decay (α decay)
1. Emission of an alpha particle from the nucleus
2. An alpha particle is simply a Helium-4 nucleus (two protons, two neutrons, but no electrons)
3. Least penetrating particle; can be stopped with a sheet of paper
ii. Beta decay (β decay)
1. Emission of an electron from the nucleus
2. A beta particle is just an electron
a. How did an electron get into the nucleus in the first place? A beta particle results when a neutron in the nucleus turns into a proton (which stays behind in the nucleus) and an electron (which gets shot out of the nucleus)
3. Beta particles are more penetrating than alpha particles; a beta particle can be stopped by a sheet of aluminum foil (but will not be stopped by a mere sheet of paper)
iii. Gamma decay (γ decay)
1. Emission of gamma radiation from the atom.
2. Radiation is just light. So, a gamma “particle” is a particle of light, which is called a photon.
3. Gamma radiation is very penetrating, and will be stopped only by lead or a similar material. Remember, gamma radiation is radiation that is even more powerful than X-rays.
b. Fission
i. Fission is the splitting of a nucleus into two smaller nuclei.
ii. Fission occurs in nuclear power plants and in the original nuclear bombs dropped on Japan.
iii. Ideal fission fuel is large atom (U or Pu)
iv. Nuclear chain reaction
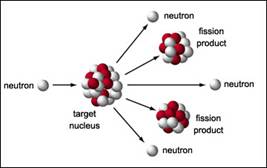
(If you are reading these notes online, click here to see a short movie depicting a nuclear chain reaction. If that link doesn’t work, consult the Documents page and look for the movie under Animations.)
1. Nuclear bombs
2. Nuclear power generation
a. Fuel
b. Moderators
c. Control rods
c. Fusion
i. Fusion is the combination, or “fusing” of two nuclei to make one bigger nucleus.
ii. Fusion occurs in the sun. Also, new nuclear bombs (the hydrogen bomb) use fusion. Scientists have not yet managed to use fusion in nuclear reactors, though they are trying.
iii. H and Li are common starting products for fusion. He is a common product.
d. Balancing nuclear equations
V. Uses of nuclear chemistry
a. Nuclear power
i. Why use nuclear power?
ii. Types of power generation used in the US
iii. Similarities between nuclear power generation and conventional fossil fuel-based power generation
iv. Differences between nuclear power generation and conventional fossil fuel-based power generation
v. Design of a nuclear power plant
vi. Safety
1. Safety record
2. Safety concerns
a. Meltdown
b. Radioactivity leaks
3. Waste disposal
a. Environmental and human health concerns
b. Transportation
c. “NIMBY”
d. Protection against theft for use in dirty bomb, etc.
b. Nuclear weapons
i. Fission bombs
ii. Fusion bombs
iii. Uranium enrichment
iv. Depleted uranium
v. Plutonium production
c. Archaeology
d. Radiochemistry
e. Cosmochemistry
VI. Questions that you might have
a. Nuclear arms
i. What’s so special about atomic bombs? Why did the US build an atomic bomb during WWII? Didn’t we have enough firepower (killing capacity) with conventional weapons?
ii. Why hasn’t anyone used an atomic bomb since WWII?
iii. Why didn’t the US drop nuclear bombs on Germany, the USSR, Korea, Viet Nam, Iraq, or Afghanistan?
iv. Why hasn’t any other country developed an atomic bomb (or have they)? How many countries have an atomic bomb? Why don’t more countries make atomic bombs and use them against us or each other?
v. How does the US determine if someone else is developing/buying/testing nuclear weapons?
vi. What is a dirty bomb?
b. Nuclear power
i. Is nuclear power dangerous?
ii. What is a nuclear reactor?
iii. Public attitudes to nuclear power
1. advocacy
2. criticism
3. impact of global warming
4. impact of rising energy prices (ca. 2002 until present)
5. impact of nuclear proliferation concerns
iv. Nuclear waste
v. Nuclear power disasters/accidents
a. Three Mile Island
b. Chernobyl
vi. Alternative reactor designs
a. Breeder reactors
b. Pebble bed reactors
c. Uranium enrichment
d. Plutonium production