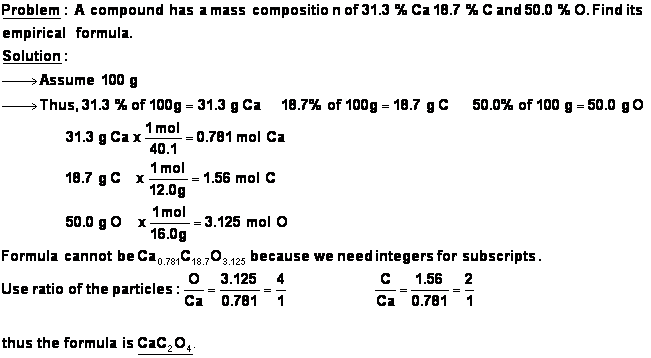Lecture Notes Chapter 6
- Introduction
![]()
- The above equation describes the synthesis of water from hydrogen and oxygen.
- It is not balanced, however.
![]()
 ŕ
ŕ 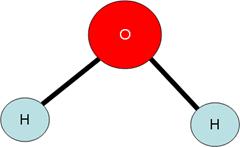
- Notice how the number of oxygen atoms on left is not equal to the number of oxygen atoms on the right.
- In order to balance these numbers out, we can not change the number of atoms in each substance. As we have seen, these formulas for the molecules are determined by the octet rule and bonding.
- Rather, we must balance the two sides of the equation by using big numbers in front of the substances in the equation. These big numbers are called coefficients.
![]()
 ŕ
ŕ 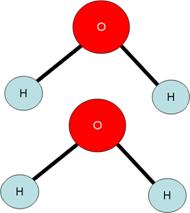
- We can see that the both sides of the equation now have the same number of blue hydrogen atoms (4). Both sides also have the same number of red oxygen atoms (2).
- The important thing to realize from this example is that chemical reactions occur by parts, not by mass. That is, when we do chemical calculations, the number of particles of each substance is often more important than the masses of the substances involved.
- For instance, the above equation DOES say that 2 molecules of hydrogen are needed to read with one molecule of oxygen in order to make two molecules of water.
- The above equation does NOT say that 2 grams of hydrogen are needed to react with 1 gram of oxygen in order to make 2 grams of water.
- Atomic mass and atomic mass units
- Atomic mass is the mass of an average atom of some element. We discussed this earlier in the year.
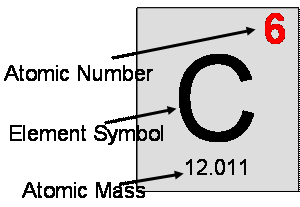
- The atomic mass is measured in small units called atomic mass units, abbreviated as amu or u. Carbon’s atomic mass is 12.011 amu.
- An amu is exactly 1/12 of the mass of a C-12 atom. This definition is the basis for the way we express the mass of any atom, not just carbon atoms.
- Atomic mass units are not really useful in the high school lab, because our instruments are not sensitive enough to detect the masses of individual atoms. It is often more convenient to measure the mass of a collection of atoms.
- The dozen is a counting number used to summarize the number of a large quantity of objects (such as cookies). We will not refer to “dozens” of atoms in this course. Instead, it will be more convenient to use moles.
- The mole
- 1 mole = 1 mol = 6.02 x 1023 things. The number “6.02 x 1023” is called Avogadro’s number, in honor of the Italian chemist Amadeo Avogadro.
- The conversion factor can be written two ways, as needed:
![]()
- A mole of objects is a huge number of objects. You could not spend a mole of dollars over your lifetime. If there are 6 billion people on Earth, and each person were to spend 3 billion dollars every day for the next 80 years, we would still not have spent 6.02 x 1023 dollars.
- The size of the mole is so large because atoms are so small. There are more atoms in a cubic inch of iron than there are grains of sand on all of the beaches on Earth.
- The particular value of the mole may seem odd to you. Why not use an even 1025, you might wonder.
- The size of the mole allows us to use the number at the bottom of each square of the periodic table as both the atomic mass (in amu) and the molar mass (in grams/mole).
- Here are some examples of converting from particles of a substance to moles of a substance, and vice versa.
Problem: convert 50 atoms of carbon to moles of carbon.
![]()
Problem: convert 50.0 moles of carbon to atoms of carbon.
![]()
- Molar mass
- The molar mass is simply the atomic mass with units of g/mol written after it.
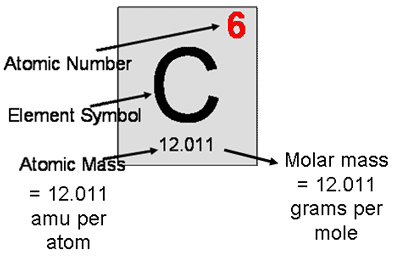
- When we say that carbon has a molar mass of 12.011 g/mol, this means that 1 mol of carbon atoms, or 6.02 x 1023 atoms, weighs 12.011 grams.
- The conversion factor can be written two ways, as needed:
![]()
- The molar mass of a compound is simply the sum of the molar masses of each of the atoms present in one molecule (or one formula unit, in the case of ionic compounds) of the compound. Consider the example of glucose (C6H12O6):
![]()
- Molar conversions
- We can covert the number of particles (atoms of an element, molecules of a molecular compound, etc.) into moles by using 6.02x 1023. Here, we convert 50.0 g of C to moles of carbon.
![]()
- Here is an example of converting from moles to grams:
![]()
Here are some practice problems:
Make the following conversions. Round answers to 3 significant figures. You will need a calculator.
- 15 apples to doz of apples
- 15 dozen apples to apples
- 20.0 mol of C to atoms of C
- 20 atoms of C to mol of C
- 10.0 lbs of apples to apples, if each apple has a weight of 0.500 lbs.
- 25 apples to lbs of apples, if each apple has a weight of 0.500 lbs.
- What is the molar mass of glucose, C6H12O6, in g/mol?
- Convert 10.0 g of glucose to mol.
- Convert 10.0 mol of glucose to g.
- Which has a greater mass, 1 mol of CO2 or 25 g of CO2?
- These types of conversions can be summarized with this chart:
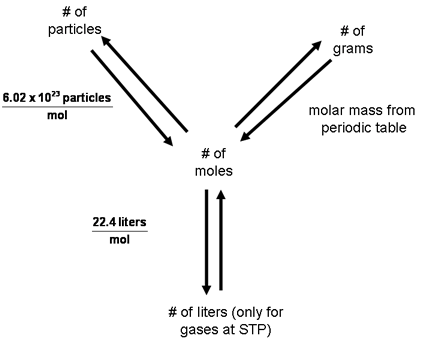
- Two steps are necessary in order to solve a problem that converts from particles to grams or vice versa:
Problem: Convert 50.0 g of H2O to molecules of H2O.
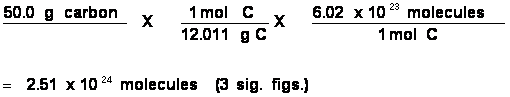
Problem: Convert 275 molecules CH4 to grams of CH4.
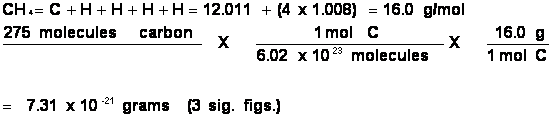
6) Converting compound formulas to % composition by mass.
a) In lab experiments this year, we will be unable to count individual numbers of atoms. Atoms are too small for this type of counting.
b) We will instead measure the masses of chemicals, and use the periodic table to convert to the numbers of moles (or numbers of particles).
c) When we are trying to determine the formula of a compound, we will need to convert the ratio of element masses to a ratio of element particles.
d) Let us first consider the easier problem of converting a coumpounds chemical formula into a mass percentage.
e) Consider water, H2O. It has two hydrogen atoms for every one oxygen atom.
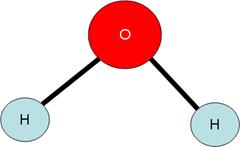
f) While it is true that 2/3 of the atoms in a molecule of water are hydrogen atoms, it is NOT true that 2/3 of the mass of a water molecule is due to hydrogen.
g) In fact, since a molecule of water weighs 18.02 amu , and since the H atoms only constitute (2 x 1.01) = 2.02 amu, the percentage by mass of the elements in water can be calculated as:
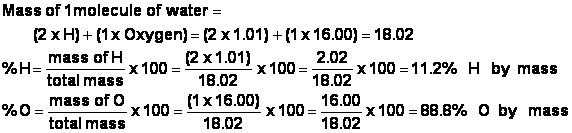
h) Here is how the mass percent of Na2CrO4 is calculated:
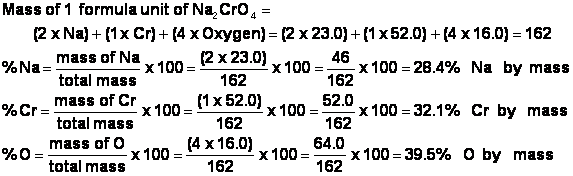
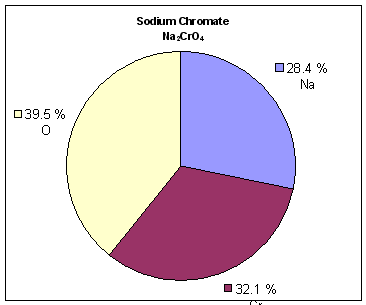
i) Here is how the mass percent of Na2Cr2O7 is calculated:
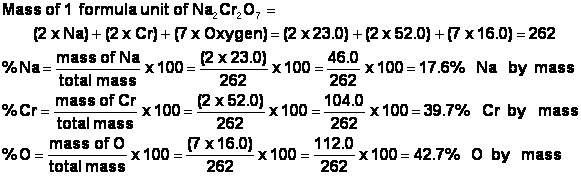
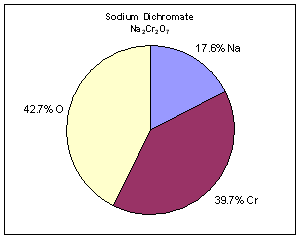
- Converting % composition by mass the empirical formula
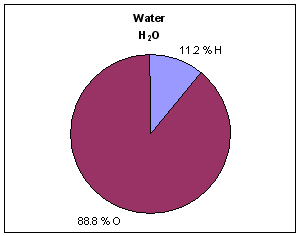
- As we have seen with the last few examples, the ratio of the masses of the elements in a compound is not the same as the ratio of number of atoms of each element in a compound. In other words, water (H2O) may be 88.8% oxygen by mass, but oxygen atoms do NOT account for 88.8% of the atoms in a glass of water.
- Let’s convert the mass % of water into the empirical formula of water. It is first necessary to turn the percentages, which are based on the masses of the elements, into the number of particles of each element.
- The Law of Definite Composition tells us that any amount of a pure substance will always have the ratios of elements as any other amount. A drop of water is 88.8% O, and a pool full of water is also 88.8% oxygen by mass.
- Therefore, to make things easy, we will assume that we have 100 g of the substance. Thus, if water is 11.2% H and 88.8% O, then 100 g of water will contain 11.2 g of H and 88.8 g of O.
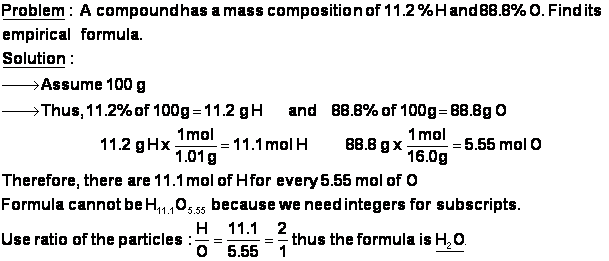
Here is another problem.