Notes, Chapters 2 &3
Matter and Energy
Properties and classification of matter
- Chemistry: the study of matter and the changes it undergoes
- Matter: anything that has mass and takes up space
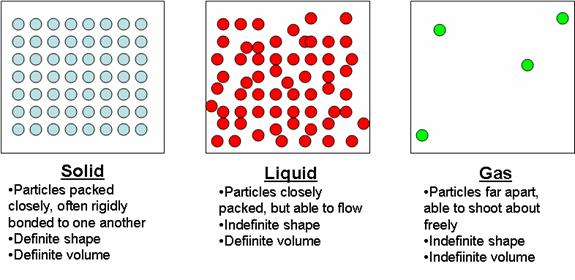
- Matter typically comes in four phases, or states:
- Solid
- Liquid
- Gas
- *Plasma
- Matter in the plasma state consists of particles in highly-excited electronic states. (Examples: the sun, a flame.) Don’t worry about plasmas for now. But just remember that 99% of the known matter in the universe is in the plasma state! (Nerd fact: we can only account for 3% of the mass of the universe – the rest is either “dark matter” . . . or a mystery! Scientists are wanted to solve this problem!)
- Physical vs. chemical changes
- Physical changes – changes in a substance that do not result in a new substance. Ex: phase changes (melting, freezing, boiling, etc.), cutting, dissolving.
- Chemical changes – changes that do result in a new substance. Ex: burning, reacting with chemicals, cooking, digesting, decomposing, spoiling.
- Chemical changes may be signaled by one or more of the following: color change, smoke formation, bubbling, change in smell. There are many exceptions to this rule, though! (ex: water bubbles when boiled, smoke appears when dry ice sublimates, etc.)
- The properties of a substance are its characteristics. Ex: properties of a fire truck are it is long, tall, red and has a loud siren.
- Intrinsic properties – properties that do not depend on amount of substance. Ex: color, density.
- Extrinsic properties – properties that do depend on amount of substance. Ex: length, mass.
- Physical properties – properties that can be observed without a chemical change. Ex: mass, volume, melting point.
· Chemical properties – properties that can not be observed without a chemical change (or attempting a chemical change). Ex: flammability, digestibility, ability to react with a certain chemical.
- Classifying matter: matter can be divided into roughly four categories according to it s make-up (i.e., composition): compounds, mixtures, elements, and solutions.
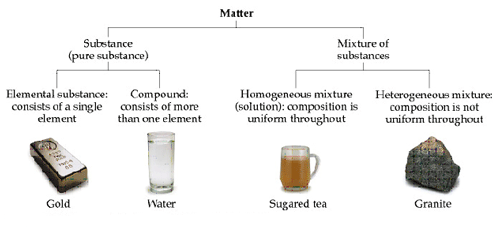
· Homogeneous: matter that appears to be the same throughout. Ex: water, gold, Kool-Aid.
· Heterogeneous: matter that does not appear the same throughout. Ex: concrete, salt and pepper mixture.
· Pure substance: a substance that can not be broken down into simpler substances by ordinary physical means. Pure substances are either compounds or elements.
· Compound: a chemical combination of two or more elements. Ex: water (H2O), table salt (NaCl).
· Element: the simplest type of pure substance. An element can not be broken down into something simpler by physical or chemical means. Ex: any element on the periodic table.
· Elements and compounds are homogeneous.
· A mixture that is homogeneous is called a solution. A solution does not need to be a liquid. Air is a solution of many gases (incl. N2 and O2), and steel is a solution of iron, carbon, and usually several other metals.
· Separation of mixtures
o We know how to combine two things in order to make a mixture.
o So . . .how can we separate a mixture into its individual parts (a.k.a. components)?
· In order to separate a mixture, we need to exploit (make use of) some difference in the properties of its components.
· Example: A plate of pennies and marbles
o You could separate them on the basis of the difference in friction between pennies and marbles (pennies won’t slide too much if you tip the plate, but the marbles will roll).
· Example: Green M&M’s among a box of M&M’s.
o Property: color (pick out the green ones by sight).
· Example: Iron filings (powder) and soap powder.
o Property: magnetism (use a magnet)
o Or . . . Property: solubility in water (dissolve the soap in water, pour away the soap/water solution, dry out the soap water solution).
· Example: sand+water
o Property: particle size (water molecules are extremely small compared to sand particles). Strategy: use filtration, which is a technique that uses special paper to hold big particles back while small particles are allowed to go through. Filter paper comes in several grades, each of which has a specific pore size.
· Example: Salt water
o Property: boiling point. The boiling point of water is ordinarily 100°C, whereas the b.p. of table salt (NaCl) is MUCH higher than that (1465°C). Separating two or more substances based on differences in their boiling points is called distillation.
o You can’t use filtration to separate a solution (in most cases). The particles of the stuff that is dissolved (the solute) are too small to be filtered, or are too close in size to the solvent particles to be separated effectively.
· Example: Alcohol + water
o Property: boiling point (B.P. of H2O = 100°C, B.P. of ethanol = 78°C) Strategy: distillation.
o Side note: drinking alcohol = ethanol. No other type of alcohol can be consumed without causing death, and even pure ethanol is a poison of you drink it more than an ounce at a time!
o Side note: ethanol is the waste product of yeast that have chowed down on sugar of some sort. They will die when the concentration of this waste product reaches 12% (12% = 24 proof). Alcohol that is stronger than 24 proof must be distilled. This is where the term “still” (distillation boiler/collector) comes from.
· Example: How would you separate a mixture of salt, sand, and water?
· Example: How would you separate a mixture of salt, sand, water, and alcohol?
|
Type of measurement |
Abbreviation |
Definition |
Units |
Instrument |
|
temperature |
T |
Average kinetic energy of an object |
degrees Celsius (°C) or Kelvins (K) |
thermometer |
|
heat |
Q |
The total amount of energy in an object due the motion of its atoms |
joules (J) or calories (cal) |
calorimeter |
· Temperature is a measure of the average kinetic energy of the particles in a substance. This means that the faster the particles in a substance move, the higher its temperature. Temperature can also be thought of as “how hot or cold an object is.”
· Heat, on the other hand, is a form of energy that takes into account not only the energy of the particles, but the number of particles as well. It takes more heat to bring a pot of boiling water to a boil than it does to bring a cup of water to a boil. However, a pot of boiling water and a cup of boiling water have the same temperature.
· Temperature is measured in degrees Celsius (°C) or in Kelvins (K).
· Heat is measured in calories (cal) or joules (J).
· A calorie is the amount of heat that is required to raise one gram of water by one degree Celsius. You need to know this.
· A calorie = 4.18 joules. You need to know this: 1cal=4.18 J
· Do example problem 3.4, p. 69.
· Don’t worry about the definition of a joule until you get to Physics class (1 J = the amount of energy needed to accelerate a 1-kg mass at a rate of 1 m/s2)
· 1 calorie is not the same as a food calorie. A food calorie, sometimes abbreviated as Cal (capital “C”) is equal to 1000 calories, or 1 kcal.
· The amount of energy needed to raise a substance by 1°C is called the heat capacity. We won’t use heat capacity.
· The amount of energy needed to raise one gram of a substance by 1°C is called the specific heat capacity. We will use specific heat capacity.
· The specific heat of water is 1cal/g°C
· Analogy: relating lifting a brick up a distance of 1 m, 2m, 3m, vs two or three bricks up a distance of 1m, 2m, 3m, etc.
· Do example problem 3.5, p. 70
· Examine Table 3.2, p. 72
· Important formula that you must know and use:

Q = heat
m = mass
C = specific heat (sometimes called specific heat capacity)
ΔT = the change in temperature, which is never to be confused with temperature
· Strangely, your book uses the formula Q=s x m x ΔT, which I’ve never seen before. The CA State end-of-course test doesn’t use this formula, either. So neither will we! J
· Do examples 3.6, 3.7, page 72 & 75.
· Elements- the basic building blocks of matter (p. 86)
o -simplest, not smallest forms of matter
o - protons (p+), neutrons (n0), and electrons (e-) are smaller than atoms, but are not simpler
o However, everyday matter is composed of atoms, not merely of subatomic particles
o “you can have a bowl full of copper atoms or oxygen atoms, but you can’t have a bowl full of electrons, for instance.”
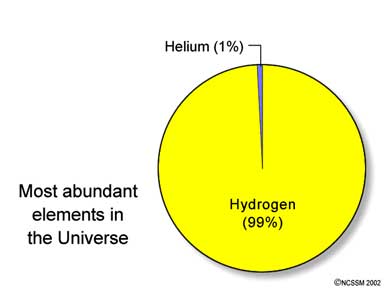
· Abundance of elements in universe (believe it or not):
· Table 4.1, p.87: Abundance of elements in earth (excl deep interior)
· Table 4.2, p.87: Abundance of elements in human body (excl deep interior)
· Element symbols (p. 89)
o Always starts with cap letter. Will sometimes have a second letter, which will always be lower case.
o Examples:
§ N = nitrogen (atomic number 7).
§ Ne= neon (atomic number 10)
§ Ni = nickel (atomic #28)
§ I = iodine (atomic # 53)
§ Bad examples: NI ¹ Ni. NE¹Ne. NE¹Ne. ne¹Ne, ne ¹Ne, etc.
o Symbols are abbreviations. Often English abbrevs., but also Latin, sometimes German or other languages that are/were international languages (see table 4.3, p. 90)
· Dalton’s Atomic theory
o Democritus: about 400 BC, Greek philosopher (NOT scientist) developed idea of all matter consisting of tiny indivisible particles.
§ Atomos: Greeak word meaning “unsplittable”
§ No experiment s to support his idea
§ Medieval idea of earth, air, fire, and water as the essential elements of all nature predominated for 2,000 years. Atomic theory known but not widely popular
o John Dalton: About 1800. England. Scientist.
§ Experiments performed on gases – had experiments to support his idea that all matter is composed of atoms.
§ The evidence: Law of constant composition
· Def:
· Ex: H2O
§ More evidence: Law of multiple composition
· Def:
· Ex: H2O and H2O2
o Dalton’s Atomic Theory (p.91)
1.
2.
3.
4.
5.
How many of these points do we believe today? If not, why not?
1.
2.
3.
4.
5.
- Democritus (Greece 400 B.C.)
· Suggested idea of atoms
· Philosopher – no experimental evidence
- John Dalton (England, early 1800’s)
· Experiments with gases
· Law of constant composition (a.k.a. law of definite proportions)
· Law of multiple proportions
· Five points of his atomic theory (p.91)
- J.J. Thomson
· Experiments on running electric current through gas discharge tubes
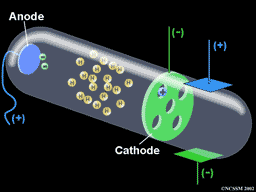
o Discovered that “rays” from one of the electrodes in the tube (the cathode) had special properties
o Concluded that these cathode rays were actually particles. Because their behavior was closely related to electric currents and fields, they were called “electrons.”
o This was called the “plum pudding” model of the atom
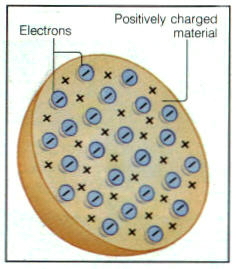
Plum pudding model of the atom
- Rutherford (England/New Zealand, 1911)
· Shot alpha particles at gold foil
· Expected that particles would mostly go through, but would occasionally be deflected
· In experiment, though, a significant number of particles bounced back from foil at extreme angles. This suggested that
§ The atom is mostly empty space
§ The mass of an atom is concentrated in a dense, positively-charged area (the “nucleus”)
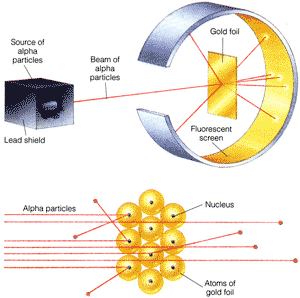
- Bohr (Denmark, 1912-13)
· Explained the emission spectrum of hydrogen
· Atoms thought to have electrons that are “orbiting” the nucleus, much as planets orbit the sun
· Electrons are in constant motion & have fixed energies that can not be decreased
· The electrons are therefore are arranged in fixed orbits at fixed distances from the nucleus
Full spectrum of visible light:

Emission spectrum of hydrogen:

Emission spectrum of iron:

o Bohr was able to explain hydrogen’s emission spectrum (H has only one electron) by reasoning that its electron was absorbing energy when it jumped to a higher energy level, and emitting energy in the form of light (a photon) when the electron relaxed back to the ground state.
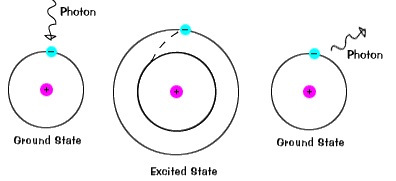
o Bohr could only describe the emission spectrum of hydrogen, not any of the spectra of more complex atoms (i.e., more than one electron per atom)
- Schrödinger, Planck, Heisenberg, Pauli
· The contributions of these scientists helped develop the quantum mechanical model of the atom (a.k.a. wave mechanical model of the atom).
· Electrons do not travel in fixed orbits, but instead travel in orbitals, which are regions in which electrons are likely to be found.
· The electrons are said to be confined to areas of probability Instead of moving in neat orbits. Several of the possible types of orbitals are shown below:
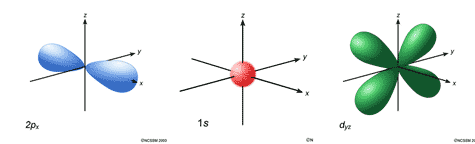
s, p, and d atomic orbitals
Further Evaluation and Homework:
· HW reviewed on Monday
· Quiz on Wednesday
· Brief review of Bohr model vs. QM model of atom, electron “orbits” vs. orbitals, ground state vs. excited state, excitation vs. relaxation, absorption of energy vs. emission of energy. What did we use to excite the atoms in Friday’s experiment? What did we use to excite the atoms in yesterday’s demonstration with the gas discharge tubes?
· Our goal in this course: to understand chemistry, which is the study of matter and the changes it undergoes.
· The way that chemical changes occur: atoms are put together, taken apart, or rearranged.
· This is achieved by the atoms giving, taking, and/or sharing electrons.
· Topics for this year:
o Which atoms give, take, or share electrons? Under what circumstances? Why these circumstances?
o How much energy is involved in these changes? Is energy required? Or is it given off? Or does it depend on the circumstances, and if so, why?
o How fast do reactions take place? Why?
o A bunch of specialized applications of chemistry: acids/bases, electrochemistry (electricity + chemistry), biochemistry, organic chemistry (carbon chem), and a whole lot of remedial physics lessons in order to understand all of the rest of these things.
· Therefore, before we talk about atoms joining with each other by doing stuff with electrons, we need to understand atoms and electrons a whole lot better.
· The atom is made of many subatomic particles. More than 200!
· We will only worry about 3 of them
o proton (p+)
o neutron (n0)
o electron (e-)
|
Subatomic Particle |
Charge |
Symbol |
Location |
Mass |
Mass |
|
proton |
+ |
p+ |
nucleus |
1.67 x 10-27 kg |
1 amu |
|
neutron |
0 (neutral) |
n0 |
nucleus |
1.67 x 10-27 kg |
1 amu |
|
electron |
- |
e- |
electron cloud |
9.109 x 10-31 kg |
1/1836 of an amu |
· Electrons are negative and protons in the nucleus are positive. They are held near to one another by the electromagnetic force. This is what causes oppositely charged particles to one another, such as when a sock sticks to your pants when you pull them out of the drier. (It’s also responsible for magnetic attractions/repulsions.)
· In the nucleus, protons are held to other protons and neutrons by the strong nuclear force. It is much stronger than the electromagnetic force.
· (Extra: there are four fundamental forces: strong nuclear force, weak nuclear force, gravitational force, and electromagnetic force. Scientists are trying to tie them altogether by creating a grand unified theory (“G.U.T.”). Albert Einstein died trying to achieve this. But then, Einstein made a good case for the argument that gravity doesn’t really exist (instead, space is curved), which is pretty much true, depending on how you define your terms. Also, if gravity really is a force, then we should be able to detect massless particles called gravitons that are associated with gravity. Hasn’t been done yet, but maybe it has, depending on how you interpret the experimental results.)
· Chemical reactions occur when we fiddle with taking electrons away from – or adding them to – the atom. This means we have to overcome the relatively weak electromagnetic force. Have you seen a bomb explode, a rocket take off, a car accelerate, or a house burn? These are examples of chemical reactions, and the energy released is due to the electromagnetic force that exists between electrons and the nucleus.
· Nuclear reactions occur when you actually break the nucleus apart . . . or when you squish particles together to make a new nucleus. The amount of energy released is much bigger in these cases. Break the nucleus apart: fission. Squeeze particles together to make a new nucleus: fusion.
· Fundere: Latin (participle: fusus) for pour/melt (fusion).
· Findere: Latin (participle: fissus) for split (fission).
· The periodic table is not arranged from lightest to heaviest atoms, though that happens to be true 95% of the time.
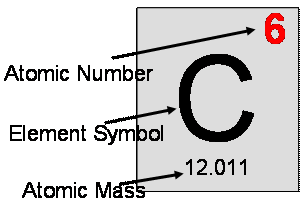
· The periodic table is arranged in order of increasing atomic number. The atomic number of an element is the number of protons in the nucleus of that atom.
· Symbol for atomic number = “Z”
·
All atoms of
a given element have the same # of protons. That is, all atoms of an element
have the same atomic number.
· The number of electrons in a neutral atom (neither a negative charge nor a positive charge) is equal to the number of protons. Positive charges and negative charges “cancel” each other out in a neutral atom.
· ** for a neutral atom** Z = p+ = e- **for a neutral atom only**
· The mass number (“A”) of an atom is the number of all of the fairly heavy stuff in an atom. That is, the mass number is equal to the number of protons plus the number of neutrons in the nucleus. The mass number of an atom of some element is NOT shown on the periodic table.
· Mass # = p+ + n0
· The mass number is shown after the element symbol and a dash. It can also be shown in the upper left corner above the atomic number:
o
A carbon atom with
a mass number of 12: ![]() or C-12
or C-12
o
A carbon atom with
a mass number of 14: ![]() or C-14
or C-14
· Example problems . . .
· What is the atomic number of each of these elements?
o iron
o cobalt
o calcium
o fluorine
o chlorine
· How many electrons would there be in the nucleus of each of the above elements? (NONE!) How many electrons outside of the nucleus?
· How many neutrons? (Can’t say!)
· Now let’s look at three different forms of the element carbon
|
|
Carbon (mass number = 12) |
Carbon (mass number = 13) |
Carbon (mass number = 14) |
|
# of protons |
6 |
6 |
6 |
|
# of electrons |
6 |
6 |
6 |
|
# of neutrons |
6 |
7 |
8 |
|
mass # |
12 |
13 |
14 |
|
symbol |
C-12 |
C-13 |
C-14 |
|
. . . or, this symbol |
|
|
|
· Atoms with the same number of protons or different numbers of neutrons are called ISOTOPES of one another. That is, atoms of the same element with different numbers of neutrons are ISOTOPES of the same element. Finally, another way of saying this is that ISOTOPES of an element have the same atomic number, but different mass numbers.
· isos (Greek, “equal”) + topos (Greek “place”)
·
Now do this on
your own (similar to next quiz):
|
|
p+ |
n0 |
e- |
mass number |
Z |
|
|
15 |
16 |
|
|
|
|
|
|
|
|
|
|
|
|
6 |
8 |
|
|
|
|
|
7 |
7 |
|
|
|
|
|
|
|
92 |
235 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
· Are there any atoms above that are isotopes of the same element?
· Are atoms with the same mass # necessarily isotopes of one another?
· The atomic mass of an element is not the same as its atomic number or its mass number. The atomic mass – which really should be called the “average atomic mass” – is not the mass of any one given atom. It is a weighted average of the mass numbers of each of the isotopes of an element.
· It is not possible to speak of the atomic mass of an isotope. It is not possible to speak of the mass number of an element.
· What is the average of 12, 13, and 14?
· Imagine that you are beginning a new course in college. If you got a 100 on a test and a zero on a quiz, would you expect your average to be 50? Why not? Are tests worth the same as quizzes in most classes?
· Example: calculating the atomic mass of carbon.
· Isotope: C-12 Abundance in nature: 98.89%
· Isotope: C-13 Abundance in nature: 1.109 %
· Isotope: C-14 Abundance in nature: 0.00000000012 %
· The average mass of a carbon atom (a.k.a. “the atomic mass”) found in a random sample of natural carbon . . .
![]()
= 12.011 a.m.u.
· You will need to do the type of calculation shown above on a quiz or a test. However, many atomic mass-related questions can be answered by inspection (without calculations). Consider this question: Hydrogen has three isotopes: H-1, H-2, H-3. What is hydrogen's atomic mass? Which of the three isotopes would you therefore expect to be the most abundant (common) in nature? Why?
· Go over review sheet: Atomic Calcs