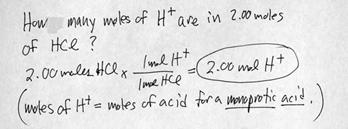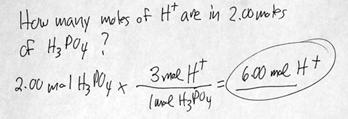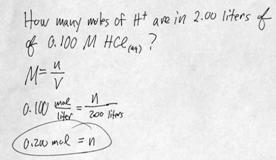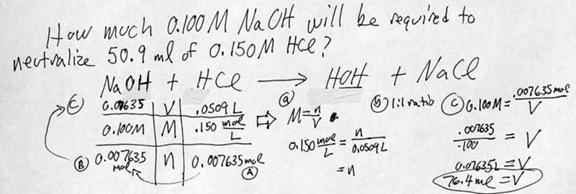Lecture Notes for Chapter 16: Acids and Bases
I. Acids and Bases
a. There are several ways to define acids and bases. Perhaps the easiest way to start is to list some of the properties of acids and bases.
b. The table below summarizes some properties that will be helpful as we learn more about acids and bases. In general, though, it is useful to say that:
i. Acids are substances which donate hydrogen and form lots of H3O+ (hydronium) ions when dissolved in water
ii. Bases are substances which accept hydrogen and form lots of OH- (hydroxide) ions in solution.
|
Property |
Acids |
Neutral Solutions |
Bases |
|
Taste |
Sour taste |
- |
Bitter taste |
|
Feel |
- |
- |
Slippery |
|
Litmus Test |
Turns blue litmus red |
Will not change the color of litmus |
Turns red litmus blue |
|
Phenolphthalein Test |
Colorless in phenolphthalein |
Colorless in phenolphthalein |
Turns phenolphthalein pink |
|
Arrhenius Definition |
Produces H+ ions in aqueous solution |
- |
Produces OH- ions in aqueous solution |
|
Brønsted-Lowry Definition |
Donates protons |
- |
Accepts protons |
|
Lewis Definition |
Electron pair acceptor |
- |
Electron pair donor |
|
Hydroxide ion concentration |
[OH-] < 10-7 M SMALL |
[OH-] = 10-7 M MEDIUM |
[OH-] >10-7 M LARGE |
|
Hydronium ion concentration |
[H3O+]> 10-7 M LARGE |
[H3O+] = 10-7 M MEDIUM |
[H3O+] < 10-7 M SMALL |
|
pH |
pH < 7 |
pH = 7 |
pH > 7 |
|
pOH |
pOH > 7 |
pOH = 7 |
pOH < 7 |
|
|
|
|
|
II. What are Acids and Bases?
a. Taste & feel
b. Are acids necessarily dangerous? And are bases therefore necessarily safe?
c. Litmus as an indicator
d. Phenolphthalein as an indicator
e. In chemistry, an indicator is a substance that changes color when the pH of its environment is changed. If you dissolve an indicator in a solution, it will change colors when the solution’s pH changes. At what pH value will the indicator change its color? Answer: it depends on the indicator.This is called the pH range of the indicator.
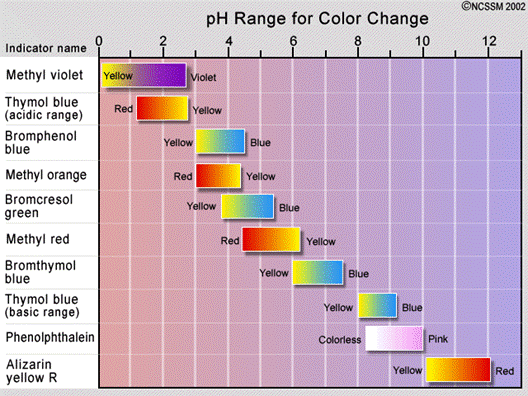
f. Arrhenius definition of acids and bases
i. Svante Arrhenius: Swedish guy
ii. Suggested that acids produced H+ ions in solution, and that bases produced OH- ions in solution
iii. Vocab moment: H+ ion is called a hydrogen ion. OH- is called a hydroxide ion.
iv. GOOD: Explained for the first time many behaviors of acids and bases, especially their ability to neutralize one another in aqueous solution:
![]()
v. SHORTCOMINGS:
1. Could not account for why some acids don’t contain H: why does CO2 produce acidic solutions when dissolved in water? Why isn’t methane (CH4) a good acid, even though it contains H?
2. Why do some bases such as NH3 (ammonia) not contain the hydroxide ion? Why can NH3 act as a base or an acid, depending on the situation?
3. ACIDS form H+ (hydrogen) ions in solution.
4. BASES form OH- (hydroxide) ions in solution.
g. Brønsted-Lowry definition of acids and bases
i. Danish guy and English guy – developed theories independently
ii. For our chemistry course, this is the most useful and important theory of acids and bases
iii. First, let’s define what we mean by “proton” when we speak of donating and accepting protons.
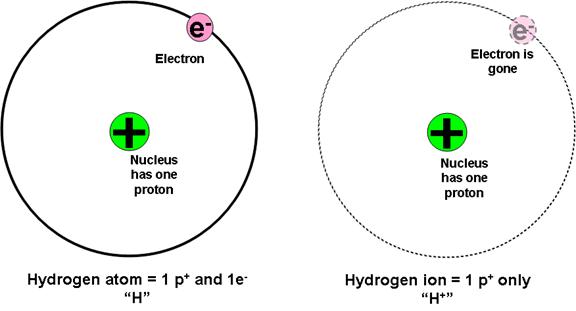
Figure 1: The difference between a hydrogen atom (H) and a hydrogen ion (H+). A hydrogen ion is the same thing as a proton.
iv. The Brønsted-Lowry definition of acids: ACIDS are proton (H+) donors.
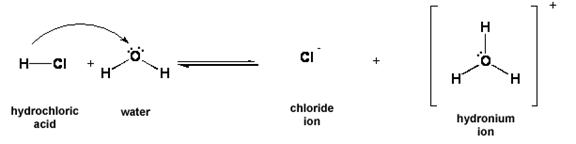
Figure 2: HCl donates H+ (a proton) to water. HCl is an example of a Brønsted-Lowry acid.
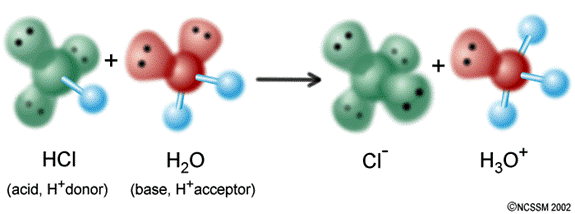
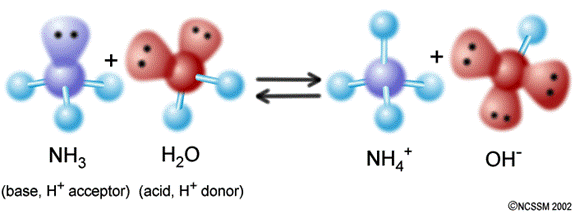
v. The Brønsted-Lowry definition of bases: BASES are proton (H+) acceptors.
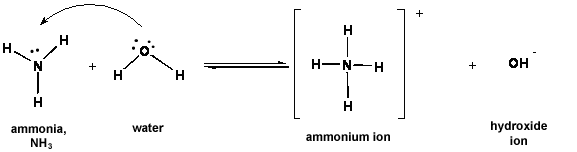
Figure 3: Ammonia (NH3) accepts a proton from water to make the ammonium ion. NH3 is an example of a Brønsted-Lowry base.
vi. Conjugate acids and bases
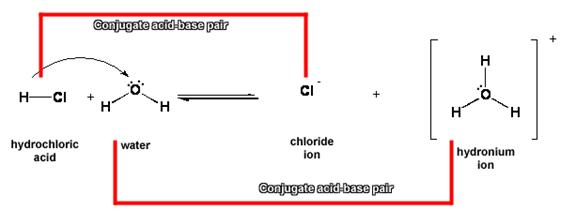
1. Substances on opposite sides of the equation that are one proton (H+) different from each other are called conjugate acid-base pairs.
2. In the reaction between HCl and water, HCl and Cl- are a conjugate acid-base pair. The other conjugate acid-base pair is H2O/H3O+.
a. HCl is the conjugate acid of Cl-, and Cl- is the conjugate base of HCl.
b. H3O+ is the conjugate acid of H2O, and H2O is the conjugate base of H3O+.
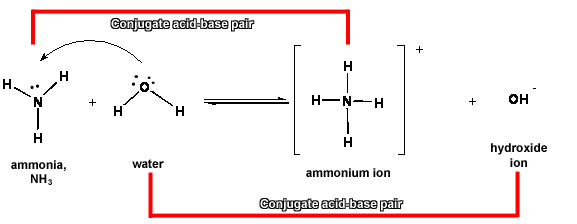
3. In the reaction between NH3 and water, ammonia/ammonium ion are a conjugate acid-base pair, and H2O/OH- are the other conjugate acid-base par.
a. NH3 is the conjugate base of NH4+, and NH4+ is the conjugate acid of NH3.
b. H2O is the conjugate acid of OH-, and OH- is the conjugate base of H3O+.
h. Lewis definition of acids and bases
i. This is the best and most current theory. However, for our chemistry course, this is the least important theory of acids and bases. The Lewis theory will be very useful in Biology class and most college chemistry classes, though.
ii. ACIDS are electron pair acceptors
iii. BASES are electron pair donors
III. The Self-Ionization of Water and the Ion Product Constant for water, Kw
a. Water can act as an acid or as a base.
b. As you can see from the reactions above, water can act as either an acid or a base, depending on the situation. Such a substance is said to be “amphoteric” or “ambiprotic”, since it can either accept or donate a proton under various conditions.
c. Pure water is not pure H2O! Can you believe it?!?! Water reacts with itself to make the OH- ion (hydroxide ion) and the H3O+ ion (hydronium ion).
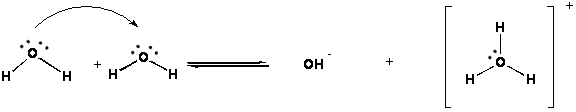
d. The concentrations of these ions in pure water at 25°C is always
[OH-] = [H3O+] = 1.00X 10-7
M
*Neutral solutions and pure water ONLY!
e. Also, we can write this chemical reaction as
2H2O (l) Û OH- (aq) + H3O+ (aq)
Therefore, we can write the equilibrium constant (Keq) expression for this reaction as
Keq = [OH-][H3O+] = [10-7 M][10-7 M] = 10-14
Notice that we have left H2O out of the Keq expression because it is a liquid.
f. This equilibrium constant has a special name; it is called the ion product constant of water. It is unitless. It is given a special symbol, too: Kw. This product will be true for any aqueous solution – not just pure water – at 25°C, which is the only temperature which we will consider. Acidic, basic, and neutral aqueous solutions always have an ion product constant that is equal to 1.00 x 10-14.
g. The following is an important formula:
Kw = 1.00 X 10-14
IV. The pH Scale
a. What is pH?
b. Introduction to the logarithm, or log10
Complete
the table below. The pH and pOH columns will be explained after the notes that
follow:
|
[OH-] |
[H3O+] |
Acidic, Basic, or Neutral Solution |
pH |
pOH |
|
1.0 X 10-7 M |
|
|
|
|
|
|
1.0 X 10-9 M |
|
|
|
|
1.0 X 10-5 M |
|
|
|
|
|
|
6.2 X 10-13 M |
|
|
|
|
9.3 X 10-9 M |
|
|
|
|
Solve for the missing value:
|
Problem |
Answer |
|
Problem |
Answer |
|
103 = ? |
|
|
10-7 = ? |
|
|
10? = 1000 |
|
|
10? = 0.0001 |
|
|
10-4 = ? |
|
|
10-3 = ? |
|
|
10? = 0.1 |
|
|
10? = 0.0368 |
|
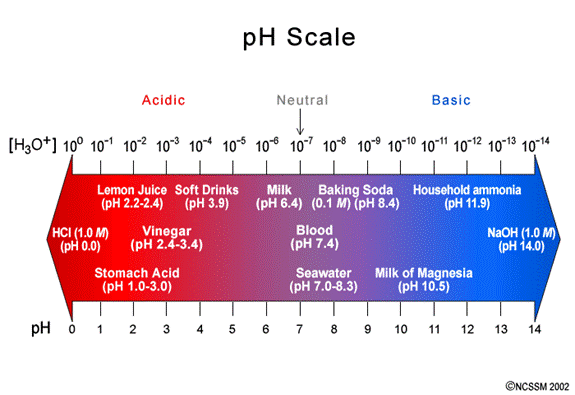
c. The pH scale is a logarithmic scale
i. This means that the pH of a solution for which [H3O+] = 0.01M is pH=2, but the pH for a solution for which [H3O+] = 0.000 001 M is pH = 6.
ii. Which solution s more acidic? Clearly the solution for which [H3O+] = 0.01M.
iii. How many times more acidic is it? Simply divide the two [H3O+] values. The answer is 0.01 ÷ 0.000 001 = 10-2 ÷ 10-6 = 10,000 times as acidic.
iv. Common mistake: many students will incorrectly say that the pH=6 solution is more acidic. In fact they will incorrectly say that it is three times as acidic.
v. Common mistake: many students will correctly say that the pH=2 solution is more acidic, but they will incorrectly say that it is three times as acidic.
vi. The pH scale is logarithmic, which is to say that pH units are each 10 times further apart from each other as you go up or down the scale. That is, DO NOT divide (pH=6) ÷ (pH=2) to determine how many times more acidic or basic one solution is than another. You should instead realize that those pH units represent powers of 10. Thus, the correct calculation is, as shown above, 10-2 ÷ 10-6.
vii. You should instead realize that a change in pH of 1 unit represents a ten-fold increase in the concentration of [H3O+], a change in pH of 2 units represents a one hundred-fold increase in the concentration of [H3O+], a change in pH of 3 units represents a one thousand-fold increase in the concentration of [H3O+],etc.
viii. The same logic is true for pOH calculations. You should instead realize that a change in pOH of 1 unit represents a ten-fold increase in the concentration of [OH-], a change in pOH of 2 units represents a one hundred-fold increase in the concentration of [OH-], a change in pOH of 3 units represents a one thousand-fold increase in the concentration of [OH-],etc.
d. The pH scale ranges from low numbers (acidic) to high numbers (basic).
e. Negative pH is possible, so is a pH = 0. (Calculate the pH of a solution of [H3O+] = 1.00 M. Calculate the pH of a solution of [H3O+] = 10.00 M.) pH and pOH values do not have to be whole numbers.
f. The further a pH value is from the neutral value of pH=7, the more acidic or basic that solution is.
i. For instance, pH=10.2 is more basic that pH=8.11.
ii. For instance, pH = 3.09 is more acidic that pH = 6.92.
g. Sometimes it is useful to keep track of pOH
h. The four important equations:
i. [H3O+] [OH-] = 1 x 10-14 = Kw
ii. pH = -log [H3O+], also written as pH = -log [H+]
iii. pOH = -log [OH-]
iv. pH + pOH = 14
i. Example problem: Determining [OH-] from [H3O+].
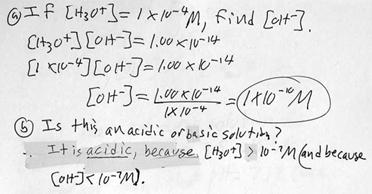
j. Example problem: Determining [OH-] from [H3O+]. This time a calculator is probably required.
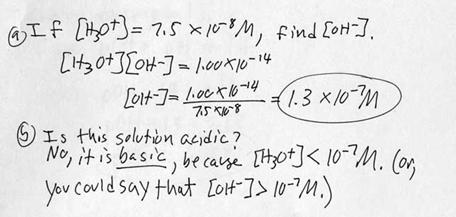
k. Example problem: Determining pH from [H3O+].
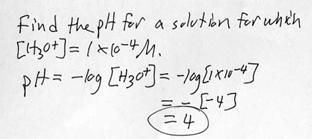
l. Example problem: Determining pH from [H3O+]. This time the concentration is not simply a power of ten, so you will need a calculator.
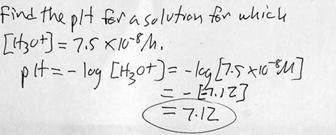
m. Example problem: Determining pOH from [OH-].
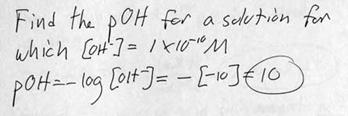
n. Example problem: Determining pOH from [H3O+].
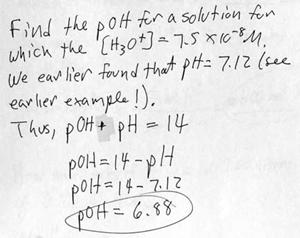
V. Acid Strength and Base Strength
a. First, it is important to emphasize the difference between the terms strong and concentrated. When a person tastes something that is very flavorful, such as a glass of concentrated Kool-Aid, he or she might remark “Wow, that’s pretty strong.” By this he or she means that there appears to be a lot of drink mix (solute) dissolved in a relatively small amount of water (solvent). As chemists, though, we would say that the Kool-Aid is concentrated, not strong.
i. CONCENTRATED = high molarity
ii. STRONG = high degree of ionization
iii. DILUTE = low molarity
iv. WEAK = low degree of ionization
b. Some acids are strong, other acids are weak.
i. Some acids, such as hydrochloric acid, will completely react with water. HCl is therefore a STRONG acid. HCl will dissociate into H+ and Cl- ions, with the hydrogen ion quickly jumping onto a water molecule to make H3O+:
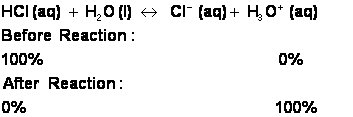
1. No matter how much you dilute HCl with water, it will always be a strong acid. If you dilute HCl, you will have created a dilute, strong acid solution.
ii. On the other hand, some acids, such as acetic acid, will only partially react with water. Acetic acid is therefore a WEAK acid. CH3COOH will dissociate into H+ and CH3COO- (acetate) ions, with the hydrogen ion quickly jumping onto a water molecule to make H3O+:
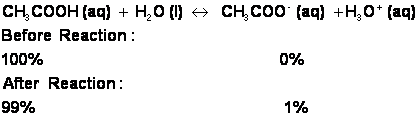
1. No matter how concentrated you manage to make a solution of acetic acid, it will always be a weak acid. If you evaporate or otherwise remove most of the water from an acetic acid solution, you will have created a concentrated, weak acid solution.
c. Base Strength
i. Bases can be either strong or weak, too.
ii. Sodium hydroxide ionizes 100% to make Na+ and OH- ions, so it is an example of a strong base.
1. No matter how much you dilute NaOH with water, it will always be a strong base. If you dilute NaOH, you will have created a dilute, strong base solution.
iii. Ammonia (NH3) reacts with water (see equation illustrated earlier in these notes) only partially. Ammonia is a weak base, just like acetic acid is a weak acid. Out of every 100 molecules of ammonia that dissolves into water, only about one has reacted to make an ammonium ion (NH4+) at any one time.
1. No matter how concentrated you manage to make a solution of ammonia, it will always be a weak base. If you evaporate or otherwise remove most of the water from an ammonia solution, you will have created a concentrated, weak base solution.
d. So, to summarize this section, we can say that STRONG ¹ CONCENTRATED, necessarily, and WEAK ¹ DILUTE, necessarily. It is possible to be both strong and concentrated, but it is also possible to be strong and dilute at the same time. It is possible to be both weak and dilute, but it is also possible to be strong and dilute at the same time. Here is a table showing examples of these combinations: