Lesson Plans Chapter 15: Solutions & Solution Chemistry
I. Solutions
a. A solution is simply a homogeneous mixture
i. Homogeneous: same throughout (it does not mean “one”) ex: water + sugar, air, alloys, aerated water
ii. Heterogeneous means different throughout (does not mean “two”) ex: oil + water
b. Solute = thing that gets dissolved (e.g., the salt in salt water)
c. Solvent = thing into which solute is dissolved (e.g., the water in salt water)
d. Special terms
i. Aqueous: refers to a solution for which the solvent is water (ex: salt water) abbreviation: “aq”
ii. Tincture: refers to a solution for which the solvent is alcohol (ex: tincture of iodine used on cuts)
iii. Alloy: a solution of two metals (bronze = copper + tin, brass = copper + zinc)
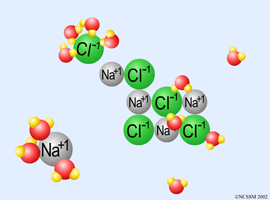
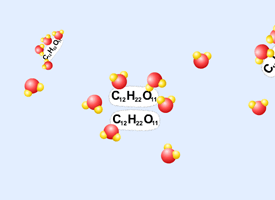
II. The Solvation Process
|
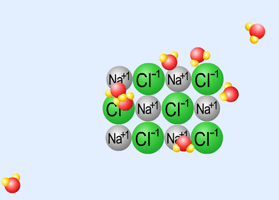
Dissolving an ionic compound in water: Table Salt (NaCl) |
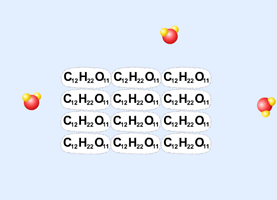
Dissolving a molecular compound in water: Table Sugar (sucrose, C12H22O11) |
|
|
|
|
|
|
|
|
|
|
|
|
I. Electrolytes and Nonelectrolytes
a. An electrolyte is a substance that will conduct electric current when it is dissolved. It will conduct electric current because it exists as charged particles (ions) when it is dissolved. Good examples: ionic compounds (such as NaCl), acids, and bases.
b. A nonelectrolyte is a substance that will not conduct electric current when it is dissolved. It will not conduct electric current because it exists as uncharged molecules when it is dissolved. Good example: table sugar (sucrose, C12H22O11).
c. Note: Ca2+, Na+, and K+ are the primary electrolytes in your body that are responsible for conducting electricity. Your body uses a lot of electricity to function – for instance, every time you move a muscle! The electricity does not travel through wires, though. It travels through your nerves.
d. Water, a molecular compound, has very few charged particles in it when it is pure. It has a natural concentration of H3O+ and OH- ions of 0.0000001 M! Other than that, it doesn’t contain any ions. Thus, pure water is a poor conductor of electricity.
e. The electrolyte will allow the bulb to light up when it’s dissolved in solution. The nonelectrolyte will not.
II. Molarity
a. Molarity is a measure of solution concentration. It is an intrinsic property, which means that concentration does not depend on the amount of a solution. For instance, if 10 g of sugar is dissolved in enough water to make a solution that has a volume of 50 ml, this solution would have the same concentration as a solution that is made by dissolving 1 g of sugar in enough water to make 5 ml of solution.
b.
![]()
![]()
c. Molarity (symbol: M) is measured in mol/L (pronounced “moles per liter”) or “M” (pronounced “molar”)
d. Example problems:
Example #1: If 3.00 moles of NaCl is dissolved in enough water to make 2.00 L of solution, find the concentration of the solution.
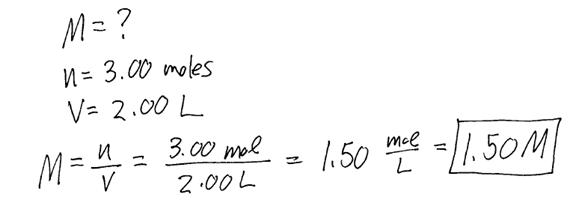
Example #2: If 3.00 g of NaCl is dissolved in enough water to make 2.00 L of solution, find the molarity of the solution.
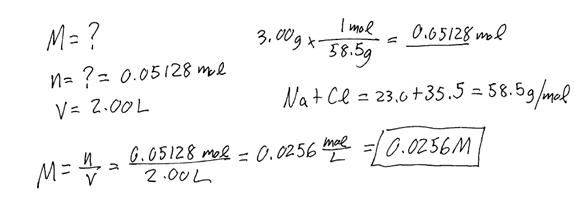
Example #3: If 3.00 g of NaCl is dissolved in enough water to make 200.0 ml of solution, find the molarity of the solution.
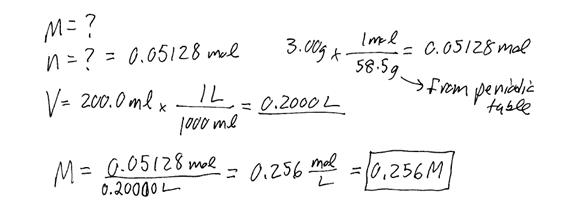
Example #4: How many mol of NaCl need to be dissolved in water in order to make 1.80 L of 1.06 M solution?
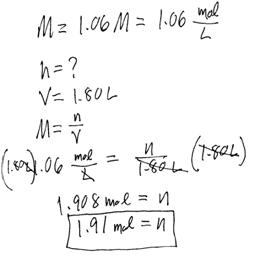
Example #5: How many g of KNO3 need to be dissolved in water in order to make 1.80 L of 1.06 M solution?
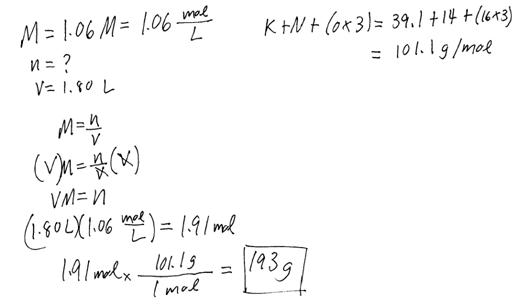
e. Other ways of expressing solution concentration
i. Percent solutions.
1. A mass % solution (%m/v) is a solution whose concentration is expressed as the number of grams of solute dissolved per 100 ml of solution
2.
![]()
3. This can be a confusing way to express concentration. It is not directly useful in stoichiometry, there are solutes which can produce %m/v values in excess of 100% m/v, and finally there are % v/v solutions, often used for liquid-in-liquid solutions.
ii. Parts per million.
1. Generally, ppm is an expression of hom many grams of a solute there are in 1,000,000 grams of the solution.
2. This is another potentially ambiguous term, because some people use “25 ppm” to mean “25 particles of solute per 1,000,000 total particles of solution” while other people use “25 ppm” to mean grams per gram or even ml per ml! To avoid ambiguity, the specific meaning of the ppm notation should be specified. (Even better, use millimolar or nanomolar!)
f. Molality: another important way of measuring concentration
i. Molality is not the same thing as molarity, even though their names are very similar.
ii. ![]()
iii. Why in the world would we have a separate way of expressing concentration, the equation for which looks so similar to the molarity equation? The answer lies in the denominator of that equation. Molality does not change with temperature of the solution. While volume of a solution can change with temperature due to expansion or contraction, the mass of the solvent will not change with temperature. For example, a sample of some aqueous solution (such as salt water) will generally expand when it is heated (volume increases) and contract when it is cooled (volume decreases). Therefore, the concentration of the solution – if you are using molarity – will change just because the temperature changed. You can avoid this confusion by computing the concentration using mass (which does not change with temperature) instead of volume. Molality, unlike molarity, does not depend on the volume of the solution.
iv. Molality is useful when you know the amount of solvent that will be added, but do not know what the final volume of the solution might be.
1. Molality is used when calculating colligative properties of a solution such as the change in freezing point, change in boiling point, or change in vapor pressure of the solution that results from adding the solute to the solvent.
2. Molality is also a convenient measure of concentration when the concentration must be computed for a liquid-dissolved-in-liquid solution.
v. Example of calculating molality
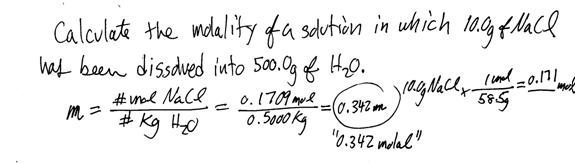
vi. Another example of computing molality

vii. Freezing point depression: an application of molality
1. The freezing point of a solution decreases with the addition of a solute
2.
![]()
![]()
a. ΔTf = change in the freezing point of the solution
b. i = the van’t Hoff factor
c. Kf = the molal freezing point constant for the solvent
d. m = molality of the solution
viii. Boiling point elevation: another application of molality
1. The boiling point of a solvent increases with the addition of a nonvolatile solute
2.
![]()
a. ΔTb = change in the boiling point of the solvent
b. Kb = the molal boiling point constant for the solvent
c. Example problem:

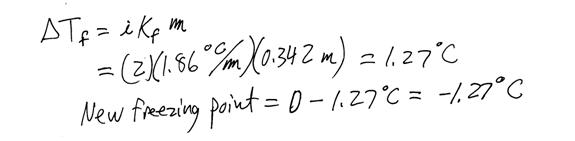
ix. The vapor pressure of a solvent also decreases as a solute is dissolved in it
1. We won’t study this effect in detail
2. Changes in vapor pressure are calculated using something called mole fraction, not molality
3. If you are interested in learning about this phenomenon, look up “Raoult’s Law.”
III. Dilutions
a. Vocabulary to know:
i. Concentrated: this means that there is a lot of solute dissolved per amount of solvent. Chemists don’t use the term “strong” in place of concentrated because “strong” has a different meaning. This will be explained in the chapter on acids and bases.
ii. Dilute: this means that there is relatively little solute dissolved per amount of solvent. “Dilute” does not mean the same thing as “weak”, a point which will be explained further in the chapter on acids and bases.
b. Why do we care about “dilutions”? Answer: Sometimes it is necessary to prepare a solution from an existing solution.
i. For instance, it is possible to prepare a solution of NaCl dissolved in water directly from the solute, because NaCl (table salt) is a solid that easily be weighed and added to water.
ii. However, HCl (aq) (hydrochloric acid) can not be prepared in a high school lab from pure HCl solute, because pure HCl (hydrogen chloride) is a highly corrosive gas.
iii. Therefore, labs usually purchase a stock solution of 12 M HCl from which the desired concentration (e.g., 0.20 M HCl) can be prepared
c.
Use this formula
to determine how to dilute a stock solution to the desired concentration:![]()
d. Justification and theory:
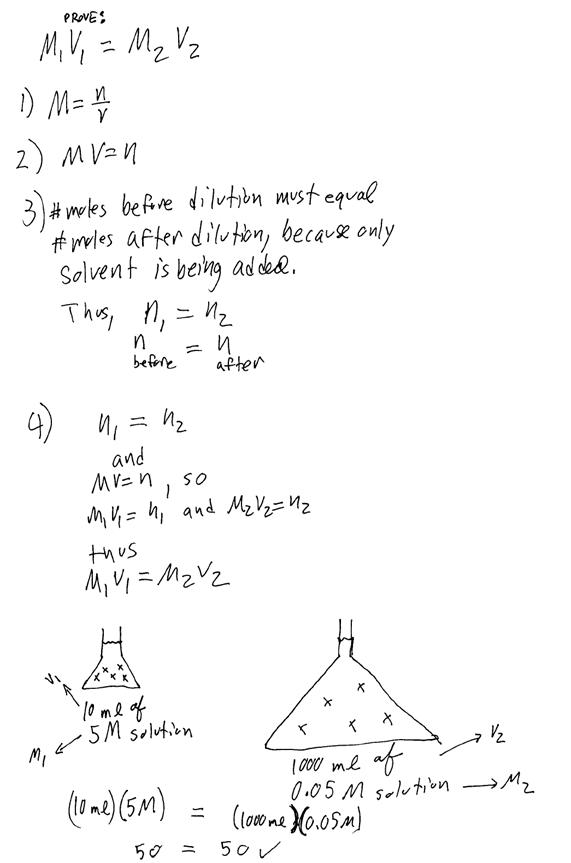
Example problem:

IV. Stoichiometry
a. Review of primitive stoichiometry: mol to mol
b. Review of mass-mass stoichiometry: g to mol, mol to mol, mol to g
c. Precipitation reactions: When a chemical reaction takes place in aqueous solution, a solid (“s”) substance that does not dissolve in water may be formed. That insoluble substance is the precipitate.
d. Neutralization reactions: When an acid and a base react in water, they will form new molecules of water and a salt.
e.
Solution
stoichiometry. This type of problem involves calculating how many ml of a
solution is needed to react with a certain number of ml of another solution.
Like all stoichiometry problems, it involves a mol-mol step that uses the
coefficients from the balanced chemical equation. However, instead of doing
gram-to-mol and mol-gram steps (which use the periodic table), there are ml-mol
and mol-ml steps that require using ![]() . Don’t forget that V must have
units of liters, because M is measured in mol/L.
. Don’t forget that V must have
units of liters, because M is measured in mol/L.
Example problem:
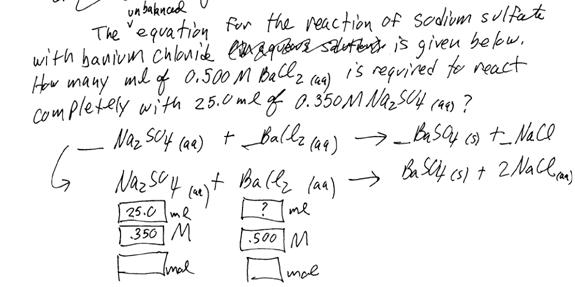
First, balance the chemical equation.
Then write down the knowns and unknowns as shown above.
Determine the number of moles of sodium sulfate that reacted first.
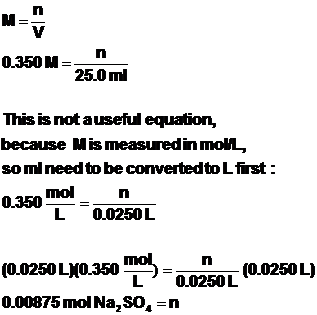
Now we use the 1:1 mol ratio of the chemical equation to compute the number of moles of barium chloride that are produced.
![]()
Now we use ![]() to
solve for the number of ml of BaCl2 needed to carry out the
reaction. Remember, the answer that comes out of the
to
solve for the number of ml of BaCl2 needed to carry out the
reaction. Remember, the answer that comes out of the ![]() equation
will be in liters, so it has to be converted to ml afterwards.
equation
will be in liters, so it has to be converted to ml afterwards.

Therefore, 17.5 ml of BaCl2 solution is needed to completely react
with 25.0 ml of 0.350 M Na2SO4.
V. Solubility
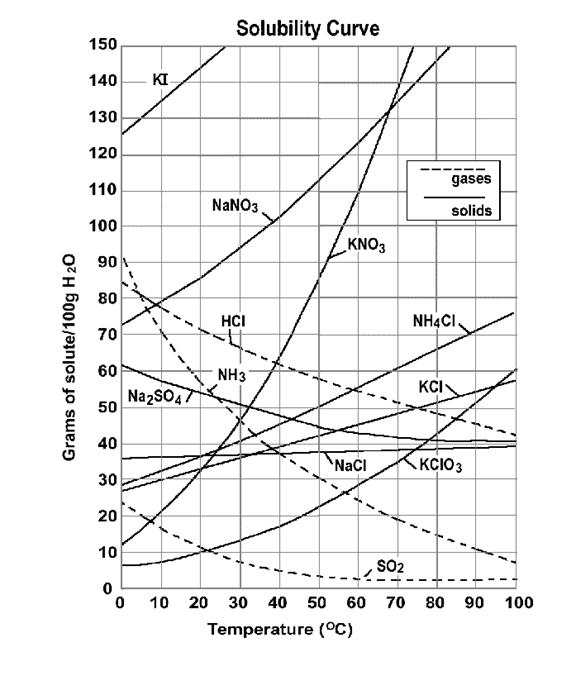
Factors Affecting Solubility:
Solubility – the amount of solute that can be dissolved in a given amount of solvent.
- usually measured in g of solute per 100 g f H2O
The nature of the solute and solvent, first and foremost, determines the solubility of the solute.
- “Like dissolves like” is the rule of thumb for solubility
o Polar solvents will dissolve polar and ionic solutes
o Nonpolar solvents will dissolve nonpolar solutes
o This is only a general rule. There are many exceptions to this rule.
Two factors that affect thedegree of solubility of a solute in a solvent
|
|
Solid in a Liquid |
Gas in a Liquid |
|
Pressure |
No effect |
P incr., S incr. (2, 3) |
|
Temperature |
T incr., S incr (1) |
T incr. , S decr. (4) |
(1) Example: Sugar dissolved in tea. In order to dissolve more sugar in tea, you should heat it up. If you dissolve lots of sugar in hot tea, then when you cool it down the sugar will re-crystallize from solution.
(2) Example: “the bends” A condition known as “the bends” occurs if you are de-pressurized too quickly from SCUBA diving. If a diver goes down very deep into the water, lots of air (a gas) will dissolve in her bloodstream. As the diver comes up to a shallow depth, the pressure decreases and the gas comes out of solution. If this re-surfacing (and de-pressurization) happens too quickly, the gas will form bubbles that block the arteries and veins of the diver, causing them to “bend” over in pain.
(3) Example: CO2 escaping from a Coke as it is opened. As pressure is decreased by opening bottle of Coke, the solubility of CO2 decreases and the gas escapes with a hiss, maybe even a fizz.
(4) Example: thermal pollution of lakes. If a lake is warmed up (by some industrial plant, for instance), the solubility of the gases (such as oxygen) dissolved in the water decreases. This can hurt animals (e.g., fish) that live in the lake.
Opposite of dissolving a solid in a liquid = re-crystallization. When conditions of a solid-in-liquid solution are changed such that its solubility decreases, the solute will come out of solutions as crystals.
Saturated – max amount of solute is dissolved in solvent (any point on the solubility curve)
Supersaturated – more than max amount of solute is dissolved in solvent (any point above the solubility curve)
Unsaturated– less than max amount of solute is dissolved in solvent (any point below the solubility curve)
Factors affecting RATE of solvation (solid in a liquid):
- Agitation (stirring) increases rate of solvation
- Decreasing particle size (grinding up solute) increases rate of solvation
- Heating increases rate of solvation
RATE of dissolving is not the same as the AMOUNT that can be dissolved.
RATE of dissolving = HOW FAST
Vs.
SOLUBILITY = AMOUNT that can be dissolved = HOW MUCH
VI. Chemical equations, complete ionic equations, and net ionic equations
We are familiar with representing chemical reactions with chemical equations. Here is the chemical equation (also called the “empirical equation” for the reaction of sodium sulfate with barium chloride in aqueous solution:
![]()
Of course, as we have seen earlier in this chapter, sodium sulfate does not exist as packages of “Na2So4” once it dissolves. Rather, it dissociates into three ions. It dissociates into two Na+ ions and one SO42- ion.
![]()
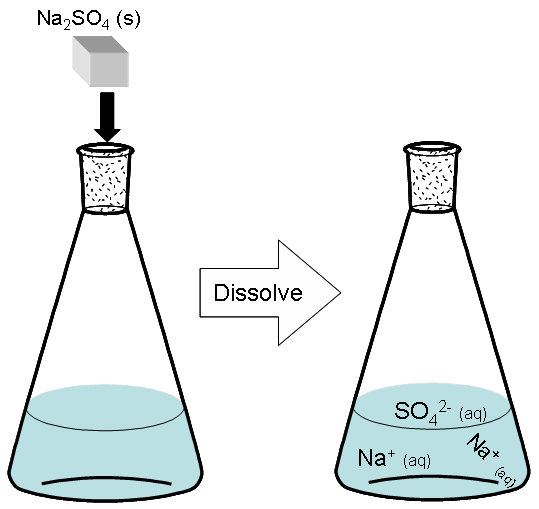
Similarly, BaCl2 does not exist as BaCl2 anymore once it dissolves in water. It dissociates into one Ba2+ ion and two Cl- ions.
![]()
Thus, when we pour together solutions of sodium sulfate and barium chloride, there are really four different ions interacting, and not simply two chemicals.
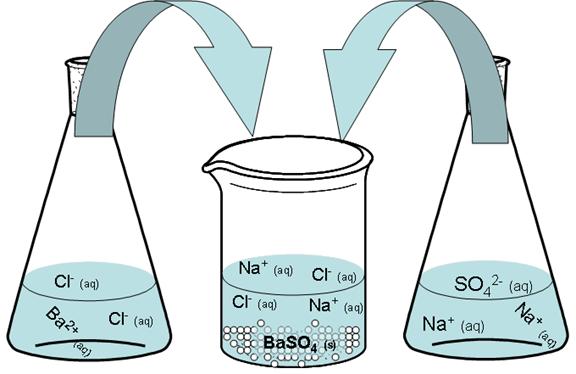
Therefore, the best way to represent this reaction is to use what is called a complete ionic equation, which shows all of the chemicals in their proper dissolved state.
“Ordinary” chemical equation:
![]()
Complete ionic equation:
![]()
Notice that the Na+ ions and the Cl- ions stay dissolved; they are “aq” before and after the reaction. However, both the barium and sulfate ions start out dissolved but end up as part of a solid (a precipitate). The ions that remain dissolved are called spectator ions.
An ionic equation that leaves out the spectator ions is called the net ionic equation.
![]()
Why did BaSO4 precipitate? How can a student predict the precipitate? Well, outside of the lab that we will do with precipitation, I will not ask you to predict precipitates. I expect you to be able to turn a chemical equation into a complete ionic equation, turn a complete ionic equation into a net ionic equation, and identify spectator ions.